A condition in which serum vitamin B12 is normal of elevated, yet and at the same time metabolic markers pr symptoms suggest that the subject is vitamin B12 deficient
Such conditions represent "Paradoxical B12 deficiency"
Metabolic analysis suggests that functional deficiency in either folate or vitamin B2 is the cause of paradoxical B12 deficiency
Resolution of Paradoxical deficiency requires complete resolution of functional B2 or folate deficiency
Paradoxical B12 deficiency is commonly seen in conditions such as
Autism
Chronic Fatigue Syndrome
Hypothyroidism
Various malignancies
Long COVID
All cause mortality
Persons with Paradoxical B12 deficiency are more likely to die from COVID-19
The current dogma on vitamin B12 deficiency implies a direct link between levels of serum vitamin B12, and sufficiency. Hence as vitamin B12 levels reduce, markers such as MMA and homocysteine increase in an inverse relationship to serum B12 levels (Minerva etal, 2021; Bailey etal, 2011). A consequence of this "Dogma" is that if a person does not have vitamin B12 levels lower than a highly variable amount, dependent upon study(148 pmol/L Minverva etal, 2021; 179 pg/ml Spain Sanz-Cuesta et al, 2012), they cannot be B12 deficient. Hence vitamin B12 deficiency would be mainly due to poor diet, or poor absorption (Elmadfa and Singer, 2009). The traditional correlation of homocystein and MMA with decreasing serum vitamin B12. This, though, is not true, elevated levels of serum vitamin B12 are very common in children with autism, and as such represent paradoxical B12 deficiency (Hope etal, 2020).
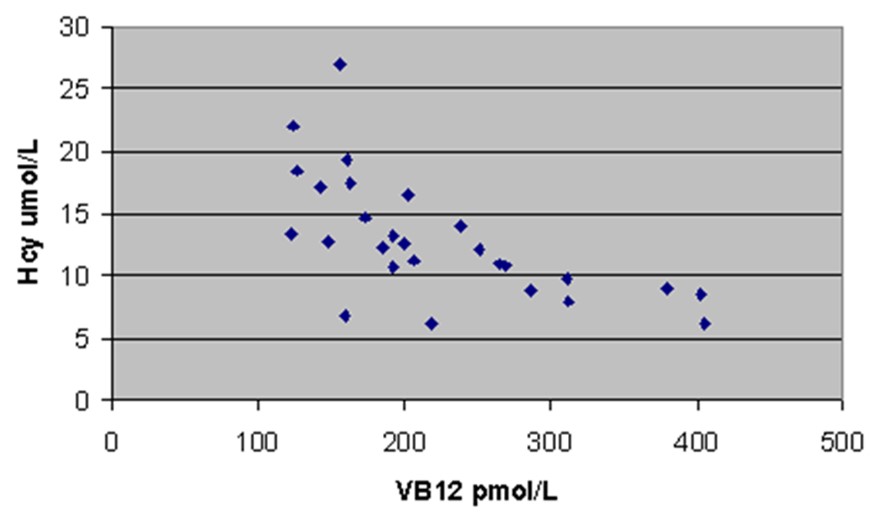
Increase in homocysteine as vitamin B12 levels decrease below 250 pmol/L from Elmadfa and Singer 2009
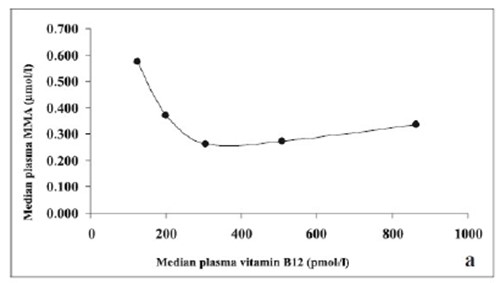
Increase in MMA as serum vitamin B12 decreases below 250 pmol/L - from Shobha etal, 2011
Many people, however, experience symptoms of vitamin B12 deficiency yet their serum levels of vitamin B12 may be normal or much higher than normal. Subsequent examination of biochemical markers such as MMA or homocysteine may show that these markers are moderate to highly elevated. As such the symptoms and biochemical markers are indicative of vitamin B12 deficiency, yet the serum vitamin B12 levels are paradoxically high. Such persons are deemed to have "Paradoxical Vitamin B12 Deficiency". Generally, however, the reason(s) for "Paradoxical B12 deficiency" are not known, even despite its association with a greater increase in "all-cause mortality" (Flores-Guerrero etal. 2020), chronic viral liver disease (Sugihara etal, 2017), Anorexia Nervosa (Corbetta etal, 2014) and death from COVID-19 .
.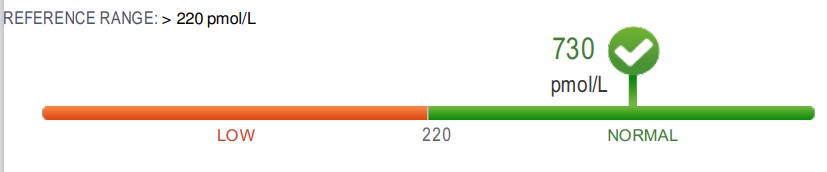
A typical example of Paradoxical B12 deficiency (as per Dynacare Plus)
It has been known for over 40 years that measurements for serum vitamin B12 levels can be greatly distorted by the presence of of non-functional cobalamin analogues (Kolhouse etal, 1978; Kane et al, 1978; Igarai et al, 1978), and in many cases measurement of serum B12 levels is not predictive of the levels of functionally active B12 (England and Linnell, 1980; Andrès et al, 2013; Serraj et al, 2011; Ermens et al, 2002; Rochat et a;. 2012l Podzolkov et a;. 2019; Zulfiqar et al, 2019). In addition, low intracellular folate levels can often be associated with the presence of inactive analogues of cobalamin in serum (Sheppard and Ryrie, 1980). Alternatively it can be associated with inflammatory conditions such as Rheumatoid arthritis due to the overproduction of the B12 binding proteins transcobalamin and haptocorin (Christensen et al 1983: Grindulis et al, 1984), Cystic fibrosis (Lindemans etal, 1984), and patients treated with nitrous oxide (Parry etal, 1985). Despite the almost completely non-predictive nature of serum vitamin B12 levels it is still the measurement of choice for vitamin B12 sufficiency. Its utility is arguably restricted to determining conditions such as dietary insufficiency, where serum levels are routinely low.In conditions such as autism, however, there is a functional deficiency in vitamin B12, yet serum vitamin B12 is generally raised (Hope etal, 2020). Little wonder that so many physicians miss functional vitamin B12 deficiency in patients!
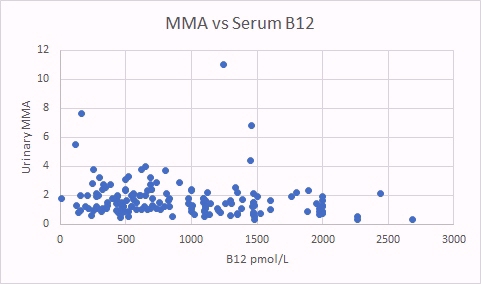
In the scattergram above there is no obvious relationship between levels of vitamin B12 in serum, and the standard marker of vitamin B12 deficiency, MMA (MethylMalonic Acid). In absolute vitamin B12 deficiency in serum, MMA starts to increase as vitamin B12 drops below 250 pmol/L.
The major cause of Paradoxical Vitamin B12 Deficiency appears to lack of functional vitamin B2, which may occur due to overt vitamin B2 deficiency in a person's diet, Hypothyrodism (Habbar etal, 2008), or due to lack of adequate intake of Iodine, Selenium and/or Molybdenum, which in turn leads to insufficient production of the two active forms of vitamin B2, namely FMN and FAD. FMN and FAD both have critical roles in cycling and maintenance of activity of vitamin B12, particularly methyl B12.
Methyl-Co(III)B12 has a major role in the body in the removal of homocysteine, and in the regeneration of methionine in the methylation cycling using the enzyme methionine synthase reductase (MTR).
In the reaction, homocysteine + Methyl-Co(III)B12[MTR] => Methionine + Co(I)B12[MTR].
The problem with this reaction is that the methyl group is lost from MethylCo(III)B12, which is reduced to Co(I)B12 and so cannot perform further methylation reactions. Theoretically if the reaction only happened once you would need approximately 13.7 gm of MethylCo(III)B12 to remethylate the 1.35 gm of homocysteine formed per day, and around 1.37 kg of the enzyme methionine synthase. Since the daily requirement for vitamin B12 is only around 5 ug, of which around 1.37 ug is MethylCo(III)B12, then clearly this does not happen.
Regeneration of MethylCo(III)B12 is performed by methionine synthase which transfers the methyl group from 5-methyl-tetrahydrofolate (5MTHF) to Co(I)B12.
Thus, 5MTHF + Co(I)B12[methionine synthase] => THF + MethylCo(III)B12[methionine synthase].
If the 5MTHF was only used once, then the body would require around 459 mg of 5MTHF per day, however, the daily requirement for folate is around 1000th of this at 400-500 ug/day, so clearly some other source, apart from diet is required to supply this amount of 5MTHF.
The solution comes from within the folate cycle. Here the THF, formed above is converted to the folate derivative 5,10-methylene-THF by the enzyme serine hydroxymethyl transferase (SHMT). The enzyme methylene-tetrahydrofolate reducate (MTHFR), then converts the 5,10-methylene group to 5-methyl-THFwhich it transports of the folate cycle into the methylation cycle, in this way a single folate molecule can be recycled over 1000 times into and out of the folate cycle providing the many 5MTHF groups for regeneration of MethylCo(III)B12.
The reaction 5,10-methylene-THF [MTHFR] => 5-methyl-THF [MTHFR]
The enzyme, MTHFR, though is critically dependent on FAD and NADPH for enzymatic activity (McNulty etal, 2014) and as levels of FAD drop, the enzyme rapidly loses activity, leading to insufficient 5MTHF for remethylation of Co(I)B12 to MethylCo(III)B12. In this instance the Co(I)B12 is rapidly oxidized to the biologically inactive Co(II)B12. The body does though have a "way around this" and it uses the enzyme methionine synthase reductase plus S-Adenosylmethionine (SAM) to remethylate Co(II)B12.
Thus, Co(II)B12[MTR] + SAM[MTRR] => MethylCo(III)B12 + SAH + MTRR.
MTRR, like MTHFR is also a "Flavoprotein" and uses both of the active forms of vitamin B2, FMN and FAD for activity. Once again the activity of the enzyme is critically dependent upon the concentration of FMN and FAD. The activity of the enzyme MTRR is so critical for regeneration of MethylCo(III)B12, that certain mutations in the gene have been found to be conditionally lethal in the womb, or are associated with much higher rates of Down Syndrome, Neural Tube Defects, increased homocysteine (Garcia-Minguillan etal, 2014; DeClerc etal, 1998) and increased cancer risks.
From the above it can readily be seen that if there is insufficient FMN and/or FAD, there will be a gradual accumulation of the inactive Co(II)B12, which is released from the cell and then starts to accumulate in serum, leading to paradoxically high serum B12.
Further, the conversion of both hydroxycobalamin and cyanocobalamin to the active Adenosyl and methyl cobalamins, also involve "Flavoproteins" (Obeid etal, 2015) and hence if a person takes or is injected with, high doses of hydroxycobalamin or cyanocobalamin and has functional B2 deficiency, the inactive hydroxycobalamin and cyanocobalamin will accumulate in serum, however the symptoms of vitamin B12 deficiency will not be resolved. Despite the obvious consequences of the functional B2 deficiency in the metabolism of B12, the majority of authors do not seem cognizant of this (Obeid etal, 2015), and seem to believe that B12 deficiency only occurs due to low intake of the vitamin, rather than ineffective processing, and are unaware of the phenomenon of Paradoxical B12 deficiency.
Paradoxical B12 deficiency is also apparent in certain cancers, such as lung cancer as a result of smoking. In this case one would expect a build up in inactive CN-Cbl due to the cyanide produced during smoking. The higher the serum B12, the higher the associated risk of lung cancer (Fanidi etal, 2019). In addition, inhalation of large quantities of nitrous oxide (Marotta and Keserwani, 2020).
The other major cause of Paradoxical B12 occurs when people take large oral doses of B12.
Normally when one either obtains vitamin B12 from digestion in the stomach or from a low dose oral B12 supplement, a protein called haptocorrin (HC), which is secreted into saliva, binds to the B12 and protects it from acid degradation in the stomach. Relatively acid resistant analogues of B12 such as cyanocobalamin have significant hydrolysis at 37oC in the acid environment of the stomach, the more sensitive methylcobalamin, is rapidly hydrolysed in stomach acid. The amount of HC, though is relatively low, and whilst it could protect around 100 ug of B12, there is not enough HC secreted to protect the B12 in some of the high dose oral supplements. Once the B12-HC complex reaches the small intestine the HC is rapidly degraded and the "protected" B12 that has been released is bound by the B12 carrier protein, Intrinsic Factor (IF). IF is relatively non-specific in its binding to B12 and so will bind both intake and hydrolysed B12. If 90%of it is degraded then 90% of the material bound by IF will also be degraded. This mix is then taken up from the intestine and bound by the B12 carrier protein, transcobalamin (TC). TC is even more promiscuous in its binding than IF and so it will transport both the intact and degraded B12 into the cell. Once inside the cell, though the two enzymes MMA-CoA mutase and methionine synthase are very specific for Adenosyl and Methyl cobalamin, respectively, and so any inactive B12 is rapidly expelled from the cells and binds to circulating haptocorrin (HC). As more and more high dose oral B12 is administered the situation gradually gets worse and worse, because now much of the HC secreted in saliva (Collins etal 1999) already has inactive B12 bound to it, and so this HC-B12 complex can no longer protect incoming B12 from acid hydrolysis. Over time, the levels of serum B12 get higher and higher, but more and more of the B12 is inactive.
From the above it can readily be seen that if levels of the two functional analogues of vitamin B2, namely FMN and FAD are reduced the following will happen.
1. The activity of MTHFR will decrease proportionally with the decrease in FAD, thus resulting in reduced production of 5MTHF.
2. The reduced 5MTHF will result in a build-up in Co(I)B12, which over time will oxidize to Co(II)B12.
3. The reduced amounts of FMN and FAD will in turn reduce the activity of the enzyme MTRR and so the levels of inactive Co(II)B12 will build up inside the cell and will eventually be discarded from the cell resulting in a build up of Co(II)B12 in serum. A condition of Paradoxical B12 Deficiency will result.
The major cause of Paradoxical Vitamin B12 Deficiency appears to lack of functional vitamin B2, which may occur due to overt vitamin B2 deficiency in a person's diet, or deficiency of Iodine, Selenium and/or Molybdenum. Such deficiencies are very common, and our studies have shown that 50% of people with CFS or ASD are deficient in Iodine, 80% in Selenium and/or 50% in Molybdenum.
Despite the fact that functional vitamin B2 as FMN and FAD is an absolute requirement for cycling of vitamin B12, this function is seldom mentioned in even the most recent review on vitamin B12 (Sobczyńska-Malefora etal, 2021), this is despite it being known for over 40 years.
See http://vitaminb12deficiency.info/hypothyroidism.htm for further information.
In cases of functional vitamin B2 deficiency, there should be a relationship between glutaric acid (a standard marker of vitamin B2 deficiency) and MMA, and other vitamin B12 deficiency markers, HVA, VMA, QA, KA and HMG. There should also be a correlation between MMA and HVA, VMA, QA, KA and HMG. However, like MMA, there should be little relationship between these markers and serum vitamin B12.
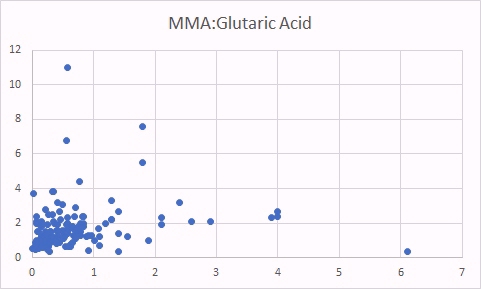
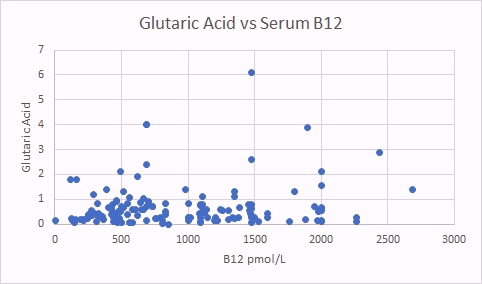
One marker of functional B2 deficiency is glutaric acid. As can be seen as glutaric acid levels increase so too does MMA, indicating reduced activity of vitamin B12. In contrast there was no relationship between glutaric acid and absolute B12 levels.
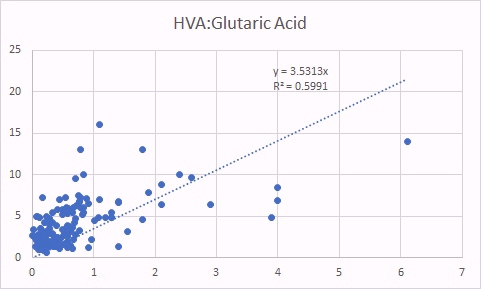
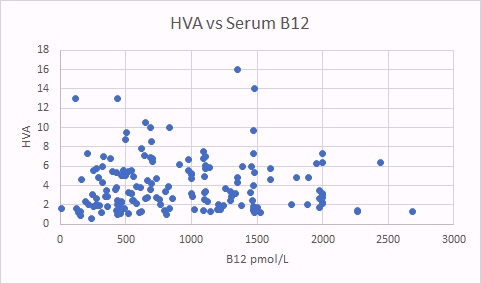
HVA is a surrogate marker for methyl B12 deficiency. There was a good correlation between HVA levels and glutaric acid, indicating that there is a correlation between increased B2 deficiency (glutaric acid increasing) and increase methyl B12 deficiency (as per the HVA marker). There was no correlation between levels of HVA and total serum B12.
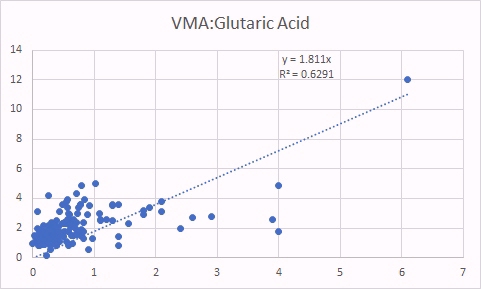
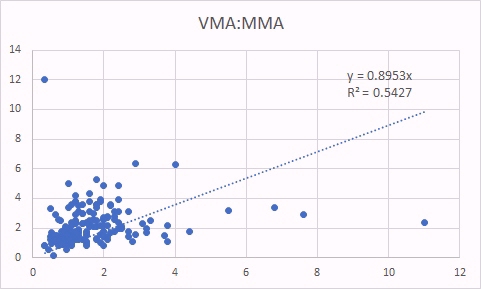
VMA is, like HVA a marker of methyl B12 deficiency. As was the case for HVA, increased VMA was correlated with increased glutaric acid (left panel) as well as increased MMA (a classical marker of B12 deficiency).

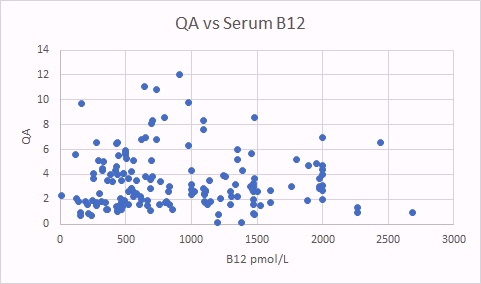
QA is also a surrogate marker for methyl B12 deficiency. There was a good correlation between QA levels and glutaric acid, indicating that there is a correlation between increased B2 deficiency (glutaric acid increasing) and increased methyl B12 deficiency (as per the QA marker). There was no correlation between levels of QA and total serum B12.
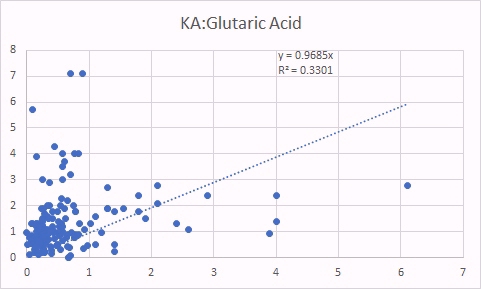
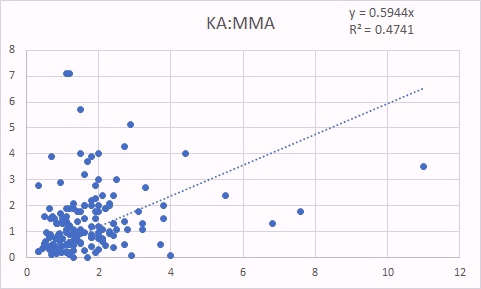
KA is also a surrogate marker for methyl B12 deficiency. The good correlation between KA levels and glutaric acid, is not as good as for QA, because you need functional vitamin B2, as FMN to form KA, so in Iodine and/or Selenium deficiency, levels of KA are reduced, and so the correlation to glutaric acid is not as good. In contrast, there was still a good correlation between KA and MMA indicating the relationship between increasing methyl B12 deficiency (KA marker) and increased Adenosyl B12 deficency (MMA marker) (Russell-Jones 2022).
The date presented above demonstrates that serum B12 levels do not correlate at all with levels of functional vitamin B12!!
More recently elevated B12 levels have been associated with a poorer prognosis following treatment for cancer (Geissbühler etal, 2000; Oh, etal, 2018; Aloreidi and Zamulko,2018; Lin etal, 2010). The higher the B12, the poorer the prognosis. We have not been able to find any study where the authors have "looked at" metabolic markers that one would ascribe to functional B2 deficiency, and compared it to the elevated B12 levels in these cancer studies. Studies on gastric cancer have suggested that the cancers themselves over-produce haptocorrin (R Binder), and hence elevated serum Hc-B12 may be indicative of prognosis (Wakatsuki etal, 1989; Lee etal, 2017; Waxman etal, 1977; Kane etal, 1978; Paradinas etal, 1982; Arendt et al, 2013; 2016; Takahashi et al, 2013). Elevations of serum B12 have also been found in cats with neoplasms (Trehy etal, 2014). Extreme levels of Hc-B12 (>18,000 pg/m B12) have been found in some metastatic cancers (Carmel, 1975) and may persist for long after the cancer has been treated, potentially indicating the presence of metastases (Lacombe etal, 2021). Studies by Russell-Jones and co-workers have shown that many cancers over-express receptors involved in vitamin B12 uptake
Chronic Fatigue Syndrome Video https://m.youtube.com/watch?v=BvEizypoyO0
Fibromyalgia
Myalgic Encephalitis
Autism
Down Syndrome
Cerebral Palsy (Bottinger etal, 2020)
Insufficient intake of vitamin B2, Iodine, Selenium and/or Molybdenum
Hypothyroidism
Rheumatoid arthritis
Cystic fibrosis
Conditions which are associated with functional B2 deficiency, or where Paradoxical B12 deficiency should be suspected
Gestational diabetes
Obesity
Diabetes
Conditions of elevated serum vitamin B12 levels above the norm.
Solid neoplasms
Myeloproliferative blood disorders
Liver diseases
Dementia
Depression
ADHD
Schizophrenia
Psychosis
Refractory treatment of vitamin B12 deficiency, particularly with hydroxocobalamin and cyanocobalamin
Chronic Viral Diseases
Lyme disease
Prolonged antibiotic treatment
Parkinson's Disease
Peripheral Neuropathy
Paresthesia
Pain and numbness in fingers or toes
Fatigue
Insomnia
Dizziness
Pain in joints
Stomach complaints
Difficulty focusing
Mood changes
Elevated homocysteine
Elevated MMA
Prolonged oral treatment with vitamin B12 in which serum B12 levels rise but there is no change in symptoms
Elevated urinary oxalates or urolithiasis
Pyroluria
Lung cancer
Short Bowel Syndrome
Elevated B12 levels have been associated with higher mortality rates in the elderly (Salles etal, 2008; Xu etal, 2021; Wolffenbuttel etal, 2020; Flores-Guerrero etal, 2021). Metabolic vitamin B12 deficiency (as is seen in paradoxical B12 deficiency) is also associated with an increased risk of dementia and stroke (Spence, 2016).
Elevated B12 levels have been associated with several liver diseases (Baker etal, 1998; Carmel etal, 2001)
Recent studies have shown that the probability of admission to ICU, of Intubation and death, rose the higher the Paradoxical B12 deficiency (Ersoz and Yulmiz 2021; Galmes et al,2020; Dalbeni et al. 2021). Paradoxical B12 deficiency, is associated with elevated homocysteine and reduced production of creatine, and the creatine break-down product creatinine.
: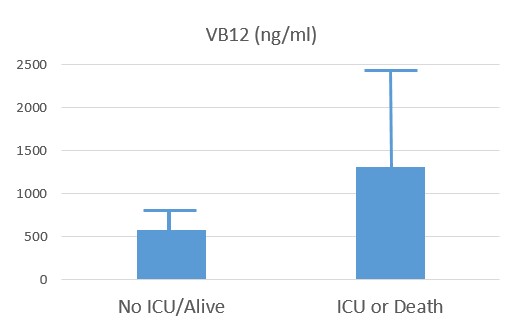
Serum Vitamin B12 in COVID-patients (Dalbeni etal, 2021)
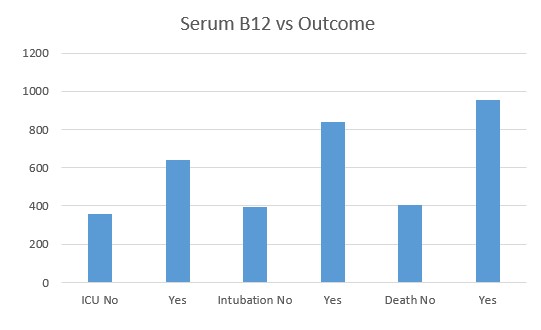
Serum vitamin B12 vs COVID deaths
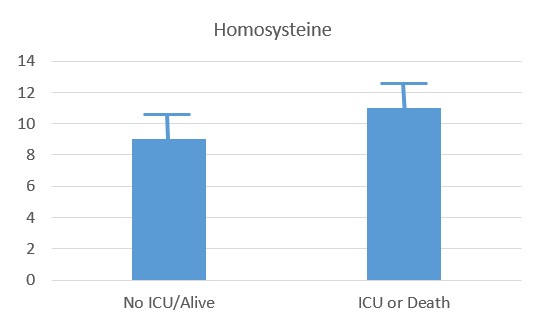
Serum Homocysteine in COVID-patients (Dalbeni et al, 2021)
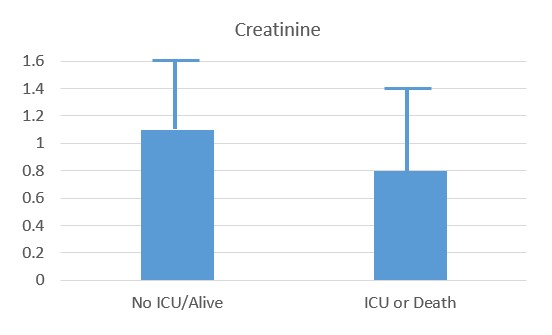
Reduced creatinine associated with ICU and Death
Paradoxical B12 deficiency can be established in a number of ways:
Measurement of serum MMA levels
Measurement of urinary MMA (Norman etal, 1982)
Organic Acids Testing, in which levels of MMA are increased, as well as HVA/VMA/QA/KA and 5HIAA, and several other markers.
Measurement of homocysteine.
Failure to diagnose paradoxical B12 deficiency can lead to "delay in the diagnosis of subacute combined degeneration of the spinal cord, and possibly permanent neurological damage" (Turner and Talbot, 2009).
Methylation is required for the production of CoQ10, with 3 methylation steps required during synthesis. In decreased methylation CoQ10 levels are lower and there is an increase in serum cholesterol
It is essential that for successful treatment of Paradoxical B12 deficiency that the cause of the functional vitamin B2 deficiency be addressed including supplementation with sufficient vitamin B2, Iodine, Selenium and Molybdenum. Such treatment, though, is generally not performed, but it has immense consequences as far as treatment results, and explains why literature on treatment of vitamin B12 deficiency is rife with examples in which supplementation studies using inactive forms of vitamin B12 (cyanocobalamin or hydroxocobalamin) without the co-administration of the necessary B2/I/Se and Mo have been ineffective in treatment. (see Langan and Goodbred, 2017;
Minerva etal Fewer adults had low or transitional vitamin B12 status..... Am J. Clin Nutr. 2021 - 1-10
Sanz-Cuesta et al Oral versus intramuscular administration of vitamin B12... PMC pub Health, 2012 12:394
Andrès, E., Serraj, K., Zhu, J., & Vermorken, A. J. (2013). The pathophysiology of elevated vitamin B12 in clinical practice. QJM : monthly journal of the Association of Physicians, 106(6), 505–515. https://doi.org/10.1093/qjmed/hct051
Shobha et al. VItamiN B12 deficiency & levels of metabolites in an apparently normal urban south Indian elderly population. Indian J. Med Res. 2011 134, 432-9 PMID: 22089603.
Hope, S., Naerland, T., Høiland, A. L., Torske, T., Malt, E., Abrahamsen, T., Nerhus, M., Wedervang-Resell, K., Lonning, V., Johannessen, J., Steen, N. E., Agartz, I., Stenberg, N., Hundhausen, T., Mørkrid, L., & Andreassen, O. A. (2020). Higher vitamin B12 levels in neurodevelopmental disorders than in healthy controls and schizophrenia: A comparison among participants between 2 and 53 years. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 34(6), 8114–8124. https://doi.org/10.1096/fj.201900855RRR
Elmadfa I, Singer I. Vitamin B-12 and homocysteine status among vegetarians: a global perspective. Am J Clin Nutr. 2009 May;89(5):1693S-1698S. doi: 10.3945/ajcn.2009.26736Y. Epub 2009 Apr 8. PMID: 1935722
Serraj, K., Mecili, M., Housni, I., & Andrès, E. (2011). Hypervitaminémie B12 : physiopathologie et intérêt en pratique clinique [Hypervitaminemia B12 (high level of cobalamin): physiopathology, role and interest in clinical practice]. Presse medicale (Paris, France : 1983), 40(12 Pt 1), 1120–1127. https://doi.org/10.1016/j.lpm.2011.08.010
Ermens, A. A., Vlasveld, L. T., van Marion-Kievit, J. A., Lensen,
C. J., & Lindemans, J. (2002). De betekenis van een te hoge
cobalamineconcentratie in het bloed [The significance of an elevated cobalamin
concentration in the blood]. Nederlands tijdschrift voor geneeskunde, 146(10),
459–464.
Rochat, M. C., Vollenweider, P., & Waeber, G. (2012). Hypervitaminémie B12:
implications cliniques et prise en charge [Therapeutic and clinical implications
of elevated levels of vitamin B12]. Revue medicale suisse, 8(360), 2072–2077.
Podzolkov, V. I., Dragomiretskaya, N. A., Dambaeva, O. T., Auvinen, S. T., &
Medvedev, I. D. (2019). Terapevticheskii arkhiv, 91(8), 160–167. https://doi.org/10.26442/00403660.2019.08.000378
Zulfiqar, A. A., Andres, E., & Lorenzo Villalba, N. (2019). Hipervitaminosis
B12. Nuestra experiencia y una revisión [Hypervitaminosis B12. Our experience
and a review]. Medicina, 79(5), 391–396.
Bailey etal. Monitoring of vitamin B12 nutritional status in
the United States by using plasma methylmalonic acid and serum vitamin B12. Am J
Clin Nutr 2011 94 : 552-61
Elmadfa and Singer vitamin B12abd homocysteine status among vegetarians: a global perspective; Am J Clin Nutrit 2009, 90 Smith and Refsum. Homocysteine - from disease biomarker to disease prevention. HIM 2021
Flores etal Association of plasma concentration of vitamin B12 with All-Cause mortality in the general population in the Netherlands JAMA 2020 3 e1919274
Sugihara etal. Falsely elevatged serum vitamin B12 levels were associated with the severity and prognosis of chronic viral liver disease. Yonago Acta Medica 2017 60:31-39
Corbetta etal Paradoxical increase of plasma vitamin B12 and folates with disease severity in Anorexia Nervosa. Int J Eat Disor 2014
McNulty etal Riboflavin lowers blood pressure in hypertensive people with the MTHFR 677TT genotype. Arch. Public Health. 2014, 72 sup K2
Kolhouse et al. Cobalamin analogues are present in human plasma and can mask cobalamin deficiency because current radioisotope dilution assays are not specific for true cobalamin. NEJM 1978 299: 785-92'
Garcia-Minguillan etal. Riboflavin status modifies the effects of methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR) plymorphisoms on homocysteine. Genes Nutr 2014, 9:435
DeClerc etal. Cloning and mapping of cHNA for methionine synthase reductase, a flavoprotein defective in patients with homocysteinuria. PNAS 95: 2059-3064
Obeid etal, Cobalamin coenzyme forms are not likely to be superior to cyano- and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency. Mol Nutr Food Res. 2015, 1364-72
Jabbar etal, 2008 Vitamin B12 deficiency common in primary hypothyroidism. J Pak Med Assoc. 2008 May;58(5):258-61
Kane et al. Vitamin B12 binding protein as a tumour marker for hepatocellular carcinoma. Gut 1978 19:1105-9
Igarai et al. Serum vitamin B12 levels of patients with rheumatoid arthritis. Tohoku JEM 1978 125:287-301
England JM, Linnell JC Problems with the serum vitamin B12 assay. Lancet 1980- 15:1072-4
Sheppard K, Ryrie D. Changes in serum levels of cobalamin and cobalamin analogues in folate deficiency Scand J. Haem. 1980 25:401-6
Collins etal Tumor imaging via Indium 111-labeled DTPA-adenosylcobalamin Mayo Clin Proc. 1999 74:687-691
Norman et al. Cobalamin (vitamin B12) deficiency detection by urinary methylmalonic acid quantitation Blood 1982 59: 1128-31
Christensen et al. Vitamin B12 binding proteins (transcobalamin and haptocorrin) in serum and synovial fluid of patients with rheumatoid arthritis and traumatic synovitis. Scand J Rheum. 1983 12:268-72
Grindulis et al, Rheumatoid arthritis: is serum vitamin B12 high in active disease? J Rheum. 1984 11:211-2
Lindemans et al. Elevated serum vitamin B12 in cystic fibrosis. Acta Paed. Scan 1984: 73: 768-71
Parry et al. Serum valine, methionine and isoleucine levels in patients anaesthetized with and without nitrous oxide. Clin Lab Haem. 1985 7:317-26
Ermens, et al. Significance of elevated cobalamin (vitamin B12) levels in blood. PMID 14636871
Marotta D A, Kesserwani H (July 21, 2020) Nitrous Oxide Induced Posterior Cord Myelopathy: Beware of the Methyl Folate Trap. Cureus 12(7): e9319. DOI 10.7759/cureus.9319
Nijhout HF, Reed MC, Budu P, Ulrich CM. A mathematical model of
the folate cycle: new insights into folate homeostasis. J Biol Chem. 2004 Dec
31;279(53):55008-16. doi: 10.1074/jbc.M410818200. Epub 2004 Oct 20. PMID:
15496403.
Agata Sobczyńska-Malefora, Edgard Delvin, Andrew McCaddon, Kourosh R. Ahmadi & Dominic J. Harrington (2021) Vitamin B12 status in health and disease: a critical review. Diagnosis of deficiency and insufficiency – clinical and laboratory pitfalls, Critical Reviews in Clinical Laboratory Sciences, 58:6, 399-429, DOI: 10.1080/10408363.2021.1885339
Langan and Goodbred. Vitamin B12 deficiency : Recognition and Management Am Fam Phys, 2017, 15, 384-389
Fanidi etal Is high vitamin B12 status a cause of lung cancer. Int J Cancer 2019 146; 1499-1503 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6642017/
Bottiger A, and Nisson TK. High levels of vitamin B12 are fairly common in children with cerebral palsy. Acta Paediatr. 2020
Kurt etal Folic Acid and vitamin B12 supplementation improves coronary flow reserve in elderly subjects with vitamin B12 deficiency Arch Med Res 2010 Jul;41(5):369-72. doi: 10.1016/j.arcmed.2010.07.007.
Khaire etal A combined supplementation of vitamin B12 and omega-3 fatty acids across two generations improves cardiometabolic variables in rats Food Funct 2016 Sep 14;7(9):3910-9. doi: 10.1039/c6fo00148c. Epub 2016 Aug 16.
Narang etal Serum Homocysteine, Vitamin B12 and Folic Acid Levels in Patients with Metabolic Syndrome J Assoc Physicians India 2016 Jul;64(7):22-26.
Adaikalakoteswari etal Vitamin B12 deficiency is associated with
adverse lipid profile in Europeans and Indians with type 2 diabetes Cardiovasc
Diabetol . 2014 Sep 26;13:129. doi: 10.1186/s12933-014-0129-4.
Al-Musharaf etal Low Serum Vitamin B12 Levels Are Associated with Adverse Lipid
Profiles in Apparently Healthy Young Saudi Women Nutrients 2020 Aug
10;12(8):2395. doi: 10.3390/nu12082395
Adaikalakoteswari et al Vitamin B12 insufficiency induces cholesterol biosynthesis by limiting s-adenosylmethionine and modulating the methylation of SREBF1 and LDLR genes Clin Epigenetics . 2015 Feb 27;7(1):14. doi: 10.1186/s13148-015-0046-8. eCollection 2015.
Pearce Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep 2004 Nov;6(6):451-6. doi: 10.1007/s11886-004-0054-3.
Dickey and Feld The thyroid-cholesterol connection: an association between varying degrees of hypothyroidism and hypercholesterolemia in women J Womens Health Gend Based Med . 2000 May;9(4):333-6. doi: 10.1089/15246090050020619.
Sampaolo etal [Relationship between hypothyroidism and cholesterol out of the records of 1756 patients]Recenti Prog Med . 2014 Feb;105(2):79-82. doi: 10.1701/1417.15701.
Russell-Jones, G.J 2022 Paradoxical B12 deficiency: Normal to elevated serum vitamin B12 with metabolic vitamin B12 deficiency. PDF https://www.iomcworld.org/articles/paradoxical-vitamin-b12-deficiency-normal-to-elevated-serum-b12-with-metabolic-vitamin-b12-deficiency.pdf
Geissbühler, P., Mermillod, B., & Rapin, C. H. (2000). Elevated serum vitamin
B12 levels associated with CRP as a predictive factor of mortality in palliative
care cancer patients: a prospective study over five years. Journal of pain and
symptom management, 20(2), 93–103. https://doi.org/10.1016/s0885-3924(00)00169-x
Oh, H. K., Lee, J. Y., Eo, W. K., Yoon, S. W., & Han, S. N. (2018). Elevated
Serum Vitamin B12 Levels as a Prognostic Factor for Survival Time in Metastatic
Cancer Patients: A Retrospective Study. Nutrition and cancer, 70(1), 37–44.
https://doi.org/10.1080/01635581.2018.1397711
Aloreidi, K., & Zamulko, A. (2018). Elevated Vitamin B12: A Rare Presentation for Gallbladder Adenocarcinoma. South Dakota medicine : the journal of the South Dakota State Medical Association, 71(4), 171–173.
Lin, C. Y., Kuo, C. S., Lu, C. L., Wu, M. Y., & Huang, R. F. (2010). Elevated serum vitamin B(12) levels in association with tumor markers as the prognostic factors predictive for poor survival in patients with hepatocellular carcinoma. Nutrition and cancer, 62(2), 190–197. https://doi.org/10.1080/01635580903305334
Wakatsuki, Y., Inada, M., Kudo, H., Oshio, G., Masuda, T., Miyake, T., & Kita, T. (1989). Immunological characterization and clinical implication of cobalamin binding protein in human gastric cancer. Cancer research, 49(11), 3122–3128.
Lee, Y. Y., Wei, Y. C., Tian, Y. F., Sun, D. P., Sheu, M. J., Yang, C. C., Lin, L. C., Lin, C. Y., Hsing, C. H., Li, W. S., Li, C. F., Hsieh, P. L., & Lin, C. Y. (2017). Overexpression of Transcobalamin 1 is an Independent Negative Prognosticator in Rectal Cancers Receiving Concurrent Chemoradiotherapy. Journal of Cancer, 8(8), 1330–1337. https://doi.org/10.7150/jca.18274<
Trehy, M. R., German, A. J., Silvestrini, P., Serrano, G., & Batchelor, D. J. (2014). Hypercobalaminaemia is associated with hepatic and neoplastic disease in cats: a cross sectional study. BMC veterinary research, 10, 175. https://doi.org/10.1186/s12917-014-0175-x
Waxman, S., Liu, C. K., Schreiber, C., & Helson, L. (1977). The clinical and physiological implications of hepatoma B12-binding proteins. Cancer research, 37(6), 1908–1914.
Kane, S. P., Murray-Lyon, I. M., Paradinas, F. J., Johnson, P. J., Williams, R., Orr, A. H., & Kohn, J. (1978). Vitamin B12 binding protein as a tumour marker for hepatocellular carcinoma. Gut, 19(12), 1105–1109. https://doi.org/10.1136/gut.19.12.1105
Paradinas, F. J., Melia, W. M., Wilkinson, M. L., Portmann, B., Johnson, P. J., Murray-Lyon, I. M., & Williams, R. (1982). High serum vitamin B12 binding capacity as a marker of the fibrolamellar variant of hepatocellular carcinoma. British medical journal (Clinical research ed.), 285(6345), 840–842. https://doi.org/10.1136/bmj.285.6345.840
Carmel R. (1975). Extreme elevation of serum transcobalamin I in patients with metastatic cancer. The New England journal of medicine, 292(6), 282–284. https://doi.org/10.1056/NEJM197502062920603
Galmés, S., Serra, F., & Palou, A. (2020). Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients, 12(9), 2738. https://doi.org/10.3390/nu12092738
Dalbeni, A., Bevilacqua, M., Teani, I., Normelli, I., Mazzaferri, F., & Chiarioni, G. (2021). Excessive vitamin B12 and poor outcome in COVID-19 pneumonia. Nutrition, metabolism, and cardiovascular diseases : NMCD, 31(3), 774–775. https://doi.org/10.1016/j.numecd.2020.12.005
Xu, K., Liu, X., Liu, J., Zhang, Y., Ding, X., Li, L., & Sun, J. (2021). Association between serum vitamin B12 and risk of all-cause mortality in elderly adults: a prospective cohort study. BMC geriatrics, 21(1), 497. https://doi.org/10.1186/s12877-021-02443-z
Lacombe, V., Chabrun, F., Lacout, C. et al. Persistent elevation of plasma vitamin B12 is strongly associated with solid cancer. Sci Rep 11, 13361 (2021). https://doi.org/10.1038/s41598-021-92945-y
Wolffenbuttel, B., Heiner-Fokkema, M. R., Green, R., & Gans, R. (2020). Relationship between serum B12 concentrations and mortality: experience in NHANES. BMC medicine, 18(1), 307. https://doi.org/10.1186/s12916-020-01771-y
Flores-Guerrero, J. L., Minovic, I., Groothof, D., Gruppen, E. G., Riphagen, I.
J., Kootstra-Ros, J., Muller Kobold, A., Hak, E., Navis, G., Gansevoort, R. T.,
de Borst, M. H., Dullaart, R., & Bakker, S. (2020). Association of Plasma
Concentration of Vitamin B12 With All-Cause Mortality in the General Population
in the Netherlands. JAMA network open, 3(1), e1919274. https://doi.org/10.1001/jamanetworkopen.2019.19274
https://m.youtube.com/watch?v=BvEizypoyO0
Salles, N., Herrmann, F., Sieber, C., & Rapin, C. (2008). High vitamin B12 level
and mortality in elderly inpatients. The journal of nutrition, health & aging,
12(3), 219–221. https://doi.org/10.1007/BF02982624
Arendt JF, Farkas DK, Pedersen L, Nexo E, Sørensen HT. Elevated plasma vitamin B12 levels and cancer prognosis: A population-based cohort study. (2016) Cancer epidemiology. 40: 158-65. doi:10.1016/j.canep.2015.12.007 - Pubmed
Takahashi K, Tsukamoto S, Kakizaki Y, Saito K, Ohkohchi N, Hirayama K. Hypercobalaminemia induced by an energy drink after total gastrectomy: a case report. (2013) Journal of rural medicine : JRM. 8 (1): 181-5. doi:10.2185/jrm.8.181 - Pubmed
Arendt JF, Pedersen L, Nexo E, Sørensen HT. Elevated plasma vitamin B12 levels as a marker for cancer: a population-based cohort study. (2013) Journal of the National Cancer Institute. 105 (23): 1799-805. doi:10.1093/jnci/djt315 - Pubmed
Baker, H., Leevy, C. B., DeAngelis, B., Frank, O., & Baker, E. R. (1998). Cobalamin (vitamin B12) and holotranscobalamin changes in plasma and liver tissue in alcoholics with liver disease. Journal of the American College of Nutrition, 17(3), 235–238.
Carmel, R., Vasireddy, H., Aurangzeb, I., & George, K. (2001). High serum cobalamin levels in the clinical setting--clinical associations and holo-transcobalamin changes. Clinical and laboratory haematology, 23(6), 365–371. https://doi.org/10.1046/j.1365-2257.2001.00134.x
Davis ET, Strogach I, Carobene M, Shaul E, Thompson J. Paradoxical Elevation of Both Serum B12 and Methylmalonic Acid Levels in Assessing B12 Status in Children With Short-Bowel Syndrome. JPEN J Parenter Enteral Nutr. 2020 Sep;44(7):1257-1262. doi: 10.1002/jpen.1764. Epub 2020 Jan 27. PMID: 31985849.
Spence JD. Metabolic vitamin B12 deficiency: a missed opportunity to prevent dementia and stroke. Nutr Res. 2016 Feb;36(2):109-16. doi: 10.1016/j.nutres.2015.10.003. Epub 2015 Oct 21. PMID: 26597770.
Turner MR, Talbot K. Functional vitamin B12 deficiency. Pract Neurol. 2009 Feb;9(1):37-41. doi: 10.1136/jnnp.2008.161968. PMID: 19151237.
Russell-Jones G, McTavish K, McEwan J. Preliminary studies on the selective accumulation of vitamin-targeted polymers within tumors. J Drug Target. 2011 Feb;19(2):133-9. doi: 10.3109/10611861003734027. Epub 2010 May 6. PMID: 20446757.
Copyright © 2018
vitaminb12deficiency.info. All Rights Reserved.
The statements on this site compose a compendium of generally recognized signs
of vitamin B12 deficiency, and problems that can then ensue They also are formulated from a summary of relevant
scientific publications. In addition they may contain some forward looking
statements of a general nature.
Reproduction in whole or in part in any form or medium without express written
permission is prohibited